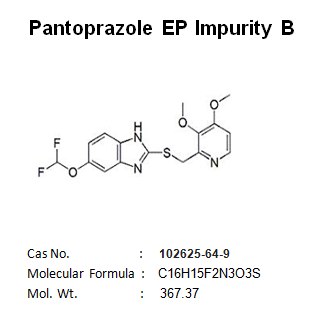
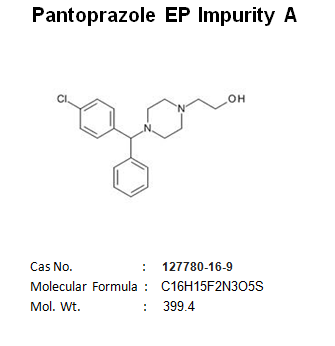
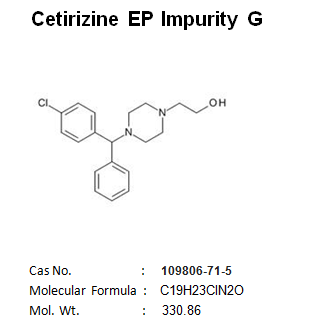
Pantoprazole EP Impurity C Manufacturer and Supplier

RXN CHEMICALS PVT LTD. situated at Pune, India which is in the business of manufacturing and supplying of Pharmaceutical Impurities, Drug Impurity, Drug Metabolites, Reference Standard Compound, and APIs We have manufacture Pantoprazole EP Impurity C . It is fully characterized using HPLC, LC-MS, 1 H NMR spectra and COA and is confirmed with RT, RRT and RF We also perform custom synthesis and purification of impurity from mg to gm scale, isolation of unknown impurity from drug product and APIs, structure elucidation as requested by our clients.From past 5 years we have been supporting all Domestic markets for Impurities & API in USA, Impurities & API in Canada and Impurities & API European countries. Rxn Chemicals can support for all Impurities which are acceptable to all regulatory agencies in the world, US-FDA, MHRA, MCC, WHO, Brazil, Japan. Rxn Chemicals is manufacturer and supplier o...